
By Vanessa Pratley
Investigative Journalist | Kaipūrongo Whakatewhatewha
Mushroom extracts are the new kid on the supplement block claiming to provide support for ills and chills. Yet our investigation found BlackGold’s Organic Mushroom Elixir may be falling foul of regulations.

BlackGold’s Organic Mushroom Elixir is a blend of powdered mushrooms designed to be taken daily, either mixed into hot drinks like coffee or added to meals.
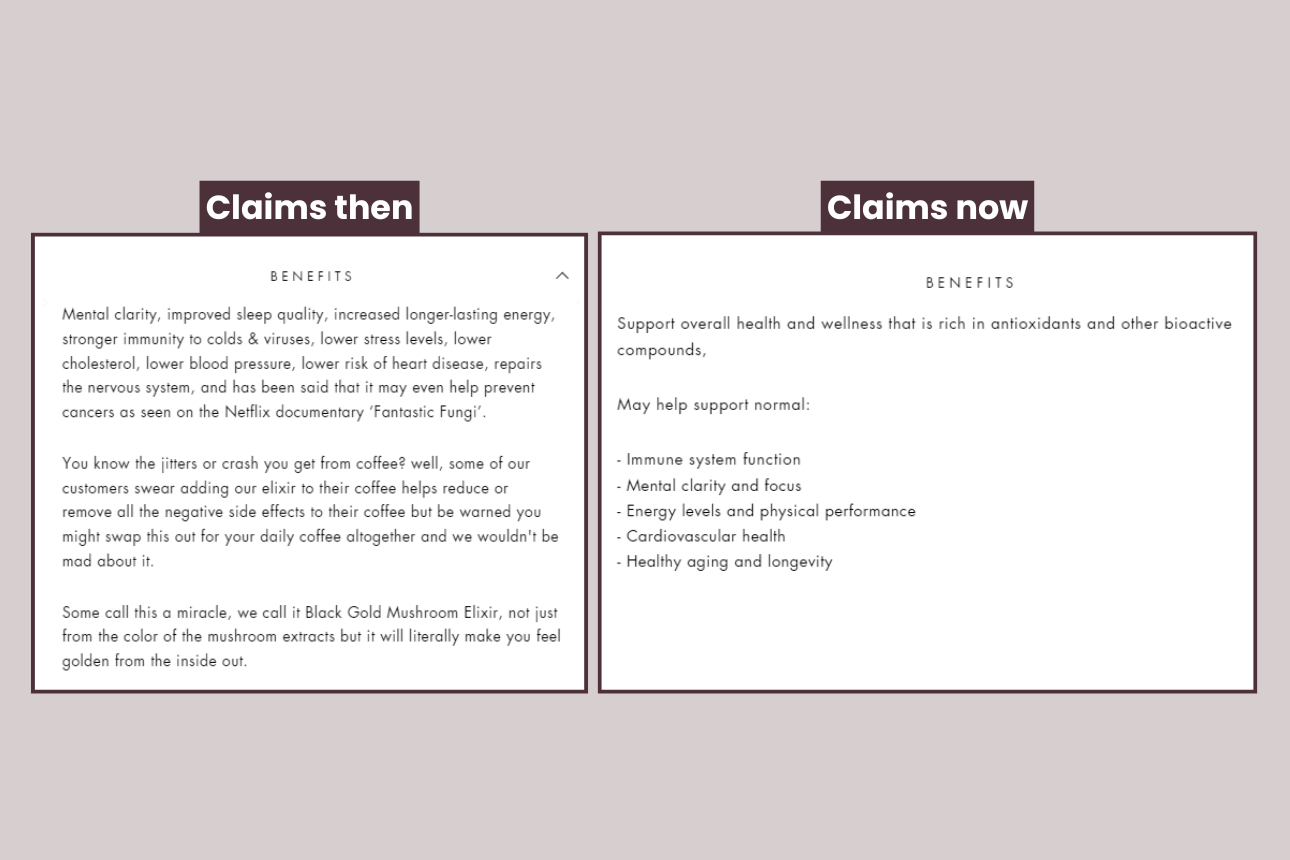
The company claims the elixir will lower cholesterol, blood pressure and stress, as well as claiming it may help prevent cancers – all for just $243 for a 6-month supply.
Its users seem pretty satisfied. After taking the mushroom elixir for 3 months, Mark said his "acute anxiety was simply gone”, according to a testimonial on BlackGold’s website.
Anita also “hasn’t looked back” after taking the elixir daily for 3 months. Not only does she sleep better than ever, but she feels sharper as well. For Tony, the elixir has done more than help his joint pain. It's also helped his inflammation and irritable bowel syndrome, and given him longer-lasting energy levels.
Although the elixir is labelled as a supplement, the statements and testimonials on BlackGold’s website make therapeutic claims, which by law only registered and pre-vetted medicines or medical devices can do.
We think BlackGold’s Organic Mushroom Elixir risks falling foul of regulations. We also think it’s yet another example of Aotearoa’s lax consumer protection for dietary supplements.
Is BlackGold’s elixir a supplement or medicine?
Based on the website, BlackGold’s Organic Mushroom Elixir sounds miraculous.
We contacted BlackGold several times asking for evidence of its claims. We also asked it to give us more information about its lab testing. We wanted to know what testing was conducted, what lab completed the testing and what the results were. BlackGold never responded to any of our questions.
A company must be able to back up its claims. It’s a requirement under the Fair Trading Act, and if a company can’t, it risks breaching the act.
Not only is BlackGold subject to the Fair Trading Act, but it’s got to abide by advertising standards too. The Therapeutic and Health Advertising Code sets out rules for advertising when it comes to medicines, dietary supplements, natural health products and other health services.
We think Blackgold risks breaching several rules, including advertising the product as magical or miraculous when it states on its website, “Some call this a miracle, we call it Black Gold Mushroom Elixir … it will literally make you feel golden from the inside out.”
Dodgy celebrity endorsements
On BlackGold’s website, underneath her own website’s logo, Nadia Lim, founder of My Food Bag, had seemingly made a statement that BlackGold’s elixir is “a symbol of longevity”. However, the statement was so vague it sounded like it could apply to any product that claimed to aid mental clarity and immunity.
So, we asked Nadia Lim if she had made the statement. It turns out, she didn’t.
A spokesperson for Nadia thanked us for bringing it to their attention. “We were not aware of this and [BlackGold does] not have our permission to use Nadia’s name.” The spokesperson said they would contact BlackGold to have the statement removed.
It took a couple of weeks, but all celebrity endorsements relating to the product disappeared. It’s likely that Vogue, Goop (Gwyneth Paltrow’s health and wellness brand) and Time never actually reviewed the product either. Unfortunately, it’s also likely that the celebrity endorsements could have led a consumer to purchase the elixir over another product. Fake celebrity endorsements can mislead consumers and using them on a website can risk breaching the Fair Trading Act.
What are regulators saying?
We presented BlackGold’s claims about the elixir to Medsafe. Medsafe is Aotearoa’s regulator of medicines and medical devices, as well as dietary supplements. We asked if the product could be considered a medicine.
A spokesperson for Medsafe said, “Some of the claims made about the product ‘Organic Mushroom Elixir’ appear to indicate that it is for a therapeutic purpose. The presentation of the product would make it a medicine.”
In other words, the product is labelled like a supplement, but the testimonials and claims imply it’s a medicine. However, there are other requirements the elixir must meet before it can be advertised as a medicine.
“Before medicines can be generally supplied or advertised, they must first be approved as medicines under the Medicines Act.”
The spokesperson confirmed that BlackGold’s mushroom elixir had not been approved.
Under the Medicines Act, anyone who sells or advertises a medicine that has not been approved may be liable to a term of imprisonment of up to 6 months or a fine not exceeding $20,000.
Medsafe didn’t elaborate on whether the elixir is a dietary supplement but noted that the product also risked breaching the Dietary Supplements Regulations.
“[The regulations] also have a requirement that therapeutic claims cannot be made,” the spokesperson said.
“Medsafe will enquire further.”
We also notified the Ministry for Primary Industries (MPI), as the body that oversees the regulation of food in New Zealand, about the elixir. We asked if it was possible the elixir should be classed as food, and if not, where it might fall in the regulatory framework.
Vincent Arbuckle, deputy director-general of New Zealand Food Safety (a business unit of MPI), said he couldn’t answer our questions. “We are looking into [this product] and are unable to provide further details.”
To file a complaint with the Advertising Standards Authority, the complainant must waive their right to complain about the advertisement to other agencies. Consumer plans to file a complaint once the other agencies we’ve approached have concluded their enquiries.

UPDATE: Following our investigation and enquiries by Medsafe, BlackGold changed the wording on its website. Instead of making therapeutic claims, BlackGold states the elixir “may help support normal immune system function, mental clarity and focus, energy levels and physical performance, cardiovascular health, healthy aging and longevity.”
Are supermarket brain drinks legit?
After New Zealand Food Safety investigated Ārepa – a blackcurrant “brain drink” that breached food standards – we decided to take a closer look at the brain drinks available at the supermarket and see if there were cheaper ways to get a boost.
Chia Sisters Brain Boost

This “Brain Boost” superfood drink boasts a blend of chia seeds and blackcurrant, designed by a neuroscientist for brain health. Its description on Woolworths’ website reads, “This is your everyday health in a bottle”. Chia Sisters recommends one to two bottles a day, but the drink costs nearly $5 per bottle.
The brain-boosting ingredient? Magnesium – required for your body to function normally, with each bottle containing 16% of your required daily intake.
But there are less expensive ways to get your daily magnesium hit. For example, you can get 14% from a single medium avocado, while two large bananas will give you 18%.
Eating a balanced diet should provide you with healthy levels of magnesium to support normal brain function. If you’re concerned about your magnesium intake, you should consult with your GP.
Because Chia Sisters attributes the brain function support claims of its Brain Boost drink to the inclusion of magnesium, it’s not in breach of the Food Standards Code, which sets rules for health claims made in relation to the food.
Snake oil? Probably not, but you can get magnesium cheaper elsewhere.
No Ugly Focus

This pretty pink “wellness tonic” claims it can help you focus. This claim is attributed to the inclusions of vitamins B5 and B12, which are necessary for normal neurological and mental function. Yet the label also notes the inclusion of ‘nootropics.’
A nootropic is a substance that can improve brain function. Nootropics in No Ugly include L-theanine, Ginkgo Biloba and Ginseng. Sounds fancy, right?
The scientific jury seems to agree that some nootropics work. For instance, amphetamines in prescription ADHD medications are effective nootropics, but there’s little evidence that Ginkgo has any proven benefits.
You don’t actually have to consume any of these substances to get your fill of a nootropic. It’s likely you’ve even had one today in the form of a tea or coffee. That’s right, caffeine is a nootropic substance, and if you’re not willing to shell out $4.80 for a bottle of No Ugly Focus, you could always perk up with a strong cup of tea.
Why therapeutic products need better regulation
The market for natural health products is lucrative. According to estimates in 2019 by industry representative Natural Health Products NZ, the sector contributes $2.3 billion annually to our economy. It’s likely this has only grown since Covid-19. Dietary supplements alone are big business.
A 2022 nationally representative Consumer survey found 51% of people took supplements or natural remedies daily. While the majority of people bought their supplements or natural remedies from the supermarket, up to 21% said they purchased theirs online.
When it came to the claims that supplements or natural remedies made, only about a third of respondents often or always researched the claims. Concerningly, 11% of people never researched the claims made, with 18% rarely doing so.
Businesses can capitalise off people who don’t do their dietary supplement due diligence. The 52% of people who trust the labels on the bottle might just be a natural remedy’s biggest customer base.
Worse than draining your wallet, supplements that illegally make therapeutic claims that don’t stack up can end up delaying proper treatment.
Consumers deserve to be able to trust product claims and despite its “100% customer happiness guarantee”, BlackGold’s Facebook is littered with comments of unhappy customers. Some complain that the product didn’t do what was advertised, while others say they never received what they ordered.
Natural health products, including dietary supplements, were set to receive stronger, fit-for-purpose regulation under the Therapeutic Products Act, passed in 2023. Belinda Castles, Consumer NZ research writer, says New Zealand’s dietary supplements regulatory regime was long overdue an overhaul, and we supported several provisions in the act, including requiring market authorisation for health products and having a permitted list of claims.
“Disappointingly, the government has announced it will repeal the act, which is a big loss for consumers as the regulations are no longer fit for purpose,” Castles said.
Without bespoke oversight, our decades-old legislation will continue to let products like BlackGold’s Organic Mushroom Elixir through the cracks, to the benefit of businesses and the detriment of consumers’ health, wellbeing and wallets.

Legal framework glossary
Medicines Act – administered by Medsafe, regulates medicines, related products and medical devices. Only approved medicines can be sold or advertised in New Zealand.
Dietary Supplements Regulations – administered by Medsafe, regulates labelling requirements and claims made by dietary supplements. Dietary supplements are prohibited under these regulations from making therapeutic claims.
Australia New Zealand Food Standards Code – administered by MPI and regulates food composition and labelling across Australia and New Zealand.
Fair Trading Act – administered by the Ministry of Business, Innovation and Employment, regulates business conduct to protect consumers, and prohibits businesses in trade from engaging in misleading or deceptive practices.
Therapeutic and Health Advertising Code – administered by the Advertising Standards Authority, sets out rules for advertising when it comes to medicines, dietary supplements, natural health products and other health services.
Why are natural health products so popular?
We asked consumers who take natural health products such as vitamins, what they take and why they take them.



